- HOME
- Business
- Clinical Stage
- Actual Development Timelines
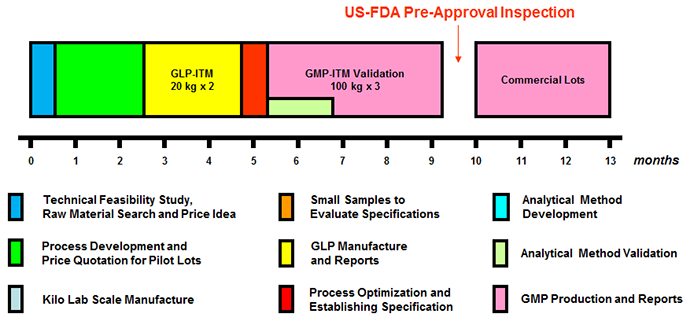
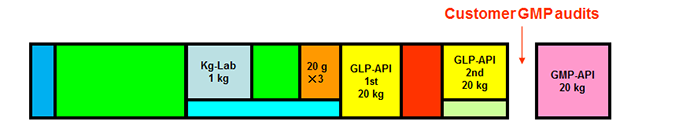
Actual Development Timeline Examples
- We manage everything under GMP from receiving raw materials to packaging the finished substance
- We have over 40 technical staff members and over 30 quality staff members who are eager to tackle any difficult situation
- We have many reactors of different types and sizes to meet your quantity demands
- We handle all validations!
- With nearly 70 years’ experience, we perform scale up studies quickly and efficiently!
Actual Example of Active Pharmaceutical Ingredient Project
Total 5 Steps, GLP-API 2 Lots, GMP-API 1 Lot
Actual Example Advanced Intermediate Project
Total 7 Steps, GLP-ITM 2 Lots, GMP-ITM 3 Lots